Abstract
Duvelisib is an orally active, dual inhibitor of phosphoinositide 3-kinase (PI3K)-δ and PI3K-γ which has shown clinical activity as a monotherapy in chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and T cell lymphoma (O'Brien ASH 2014; Flinn ASH 2014; Horwitz ASH 2014) and is being developed broadly for the treatment of B cell malignancies. Recent publications have demonstrated that PI3K-δ inhibition decreases immunosuppressive regulatory T cells (Tregs) in the tumor microenvironment (Ali, Nature, 2014; Ahmad, Cancer Res 2017) whereas PI3K-γ inhibition decreases immunosuppressive myeloid cells (MDSCs and M2 tumor-associated macrophages) (Kaneda, Nature, 2016; De Henau, Nature, 2016). Accordingly, duvelisib has recently been shown to reduce tumor and spleen MDSCs in a preclinical tumor model (Davis, Cancer Res 2017). These data collectively suggest that reduction of Tregs and immunosuppressive myeloid cells in the tumor microenvironment may contribute to the activity of duvelisib as a monotherapy, and may also augment the efficacy of immune checkpoint or co-stimulatory antibodies.
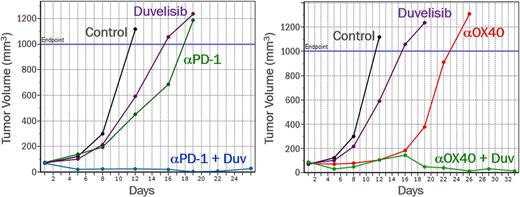
These findings led us to investigate the efficacy of duvelisib (50 mg/kg po, BID) in combination with immune checkpoint (anti-PD-1, RMP1-14) or co-stimulatory (anti-OX40, clone OX86) antibodies. In the murine syngeneic A20 B cell lymphoma model, duvelisib and anti-PD-1 treatments each induced tumor growth delay. When duvelisib and anti-PD-1 were combined in mice bearing pre-existing A20 tumors, strong synergy was observed in the anti-tumor response (see figure). Similarly, duvelisib and anti-OX40 each induced tumor growth delay as single agents in the A20 model. When anti-OX40 and duvelisib were combined, striking tumor regression was observed (see figure). To assess immune memory, tumor-free mice following anti-OX40 alone or anti-OX40 + duvelisib combination were injected with A20 lymphoma cells in the contralateral flank with no further treatment. Whereas the mice that had received anti-OX40 alone grew new tumors upon A20 re-challenge, all tumor-free mice that had received the anti-OX40 + duvelisib combination did not grow A20 tumors upon re-challenge and showed elevated memory T cells in the blood and spleen. These findings demonstrate that the anti-OX40 + duvelisib treatment established immune memory, potentially contributing to the observed tumor regression.
These data indicate that duvelisib treatment stimulates anti-tumor immunity. Furthermore, the unique dual inhibition of PI3K-δ and PI3K-γ may make duvelisib especially effective in enhancing the anti-tumor efficacy of immune checkpoint and co-stimulatory antibodies. These data support further exploration of duvelisib in combination with anti-PD-1/PD-L1 or co-stimulatory antibodies in patients with B cell malignancies.
Pachter: Verastem, Inc.: Employment. Weaver: Verastem, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.